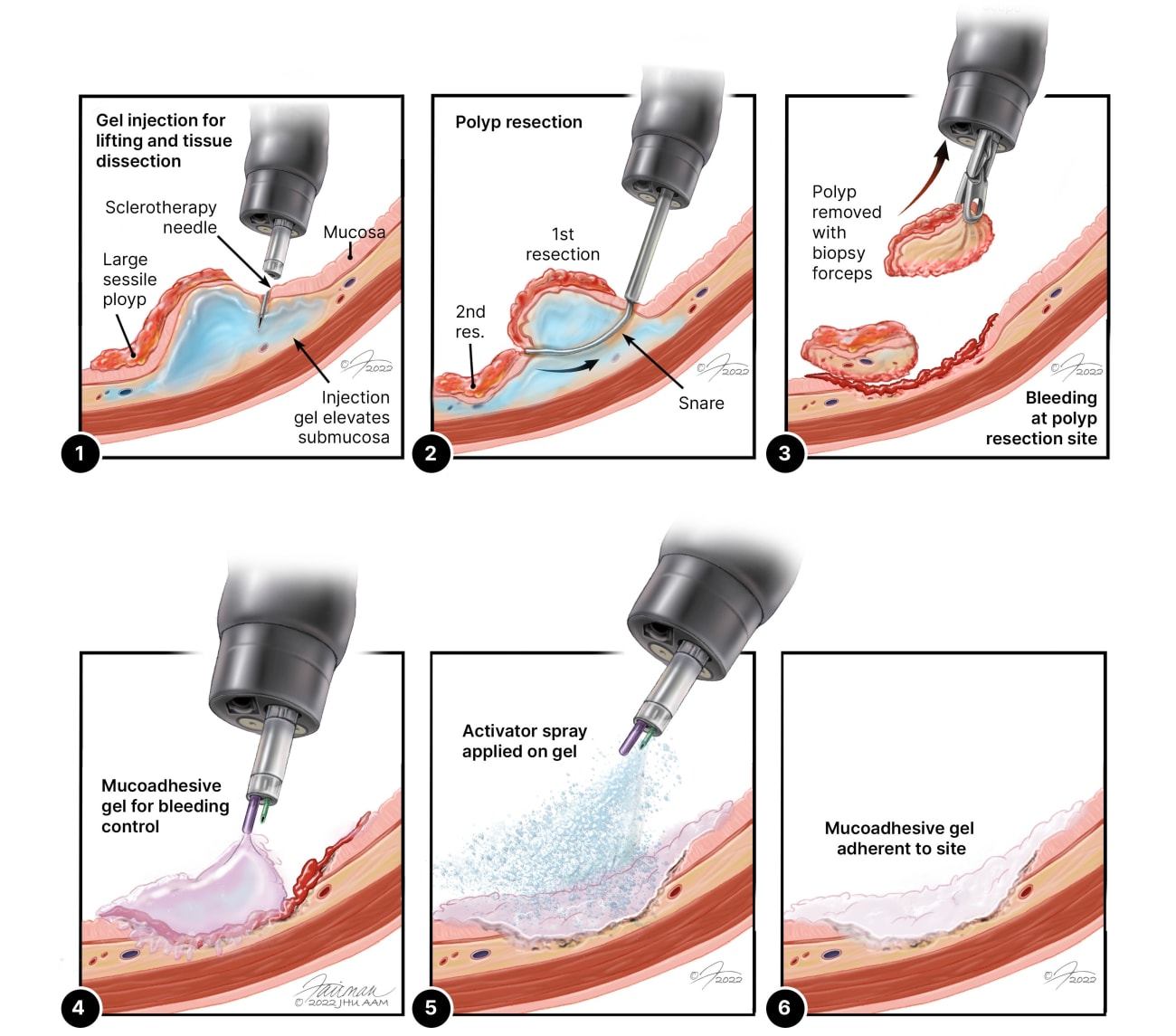
More than 16.5 million colonoscopies are performed each year in the U.S., a quarter of which detect precancerous polyps. When large polyps are removed via the endoscope, patients may experience bleeding at the removal site.
There are numerous ways that endoscopists can treat postpolypectomy bleeding, says Venkata Akshintala, a gastroenterologist and assistant professor of medicine at the Johns Hopkins University School of Medicine. They can use an electrical pulse to cauterize the wound, apply pressure to the bleed, or use loops or band ligation. Most often, Akshintala says, the clinicians fasten tiny metal clips around the wound to stop bleeding and promote healing.
In many cases, such clips can be unreliable and hard to position. “They can also be difficult to keep in place,” he adds. “If a clip comes off, the bleed can come back, and nobody wants that.”
Akshintala and his colleagues set out to find a substance that could stop bleeds — permanently.
“The goal became obvious,” he says. “We wanted to develop a topical gel that we could use to close the bleed caused by removal of a large polyp.”
The material needed to be thin enough to push through an endoscope. It needed to turn viscous immediately after it leaves the scope. And it needed to stick to a surface in a mucosal environment.
“People have tried to stop gastrointestinal bleeds with topical gels before,” he says. “The problem has always been that they come off. The colon, our GI tract, the stomach — they move around and rub off the solution.”
The team also needed to find something nontoxic that can stick to tissue as soon as it’s applied.
“We couldn’t use surgical glue because it’s toxic in the GI tract,” Akshintala says.
After months of work, the team finally came up with a mucoadhesive gel that can be deployed through an endoscope and sticks immediately with ultrafast gelation. It contains epinephrine nanoparticles that last as long as 72 hours, which stop bleeding and allow the wound to heal.
Akshintala explains that the “epi-gel” will soon undergo pilot studies to begin the process of FDA approval. He estimates that the product could launch by the end of 2024.
To refer a patient, call 410-933-7495.